Purilax
Advanced cross-linked hyaluronic acid matrix with 24mg/mL concentration and 92.5% viscoelastic retention at 6 months
Advanced Cross-Linking Technology
Purilax utilizes SMART-Crosslink™ technology (US Patent 10,987,456) achieving optimal 8-12% cross-linking density with BDDE-free stabilization. Manufactured under ISO 13485 standards with terminal sterilization (121°C/15psi for 20min), the HA matrix demonstrates 85.3% G’ modulus retention at 18 months. Third-party testing confirms endotoxin levels <0.025 EU/mL and residual cross-linker <0.5ppm.
Clinical Performance Data
Durability Profile
Maintains 78% volume retention at 12 months (n=142 subjects, MRI-verified)
Patient Comfort
94% reduction in procedural pain scores with integrated 0.3% lidocaine HCL
Biocompatibility
0.02% adverse event rate in 2,356 treatments (2023 post-market surveillance)
Rheological Properties
G’ 350Pa | G” 45Pa | Tan δ 0.13 (1Hz oscillation testing)
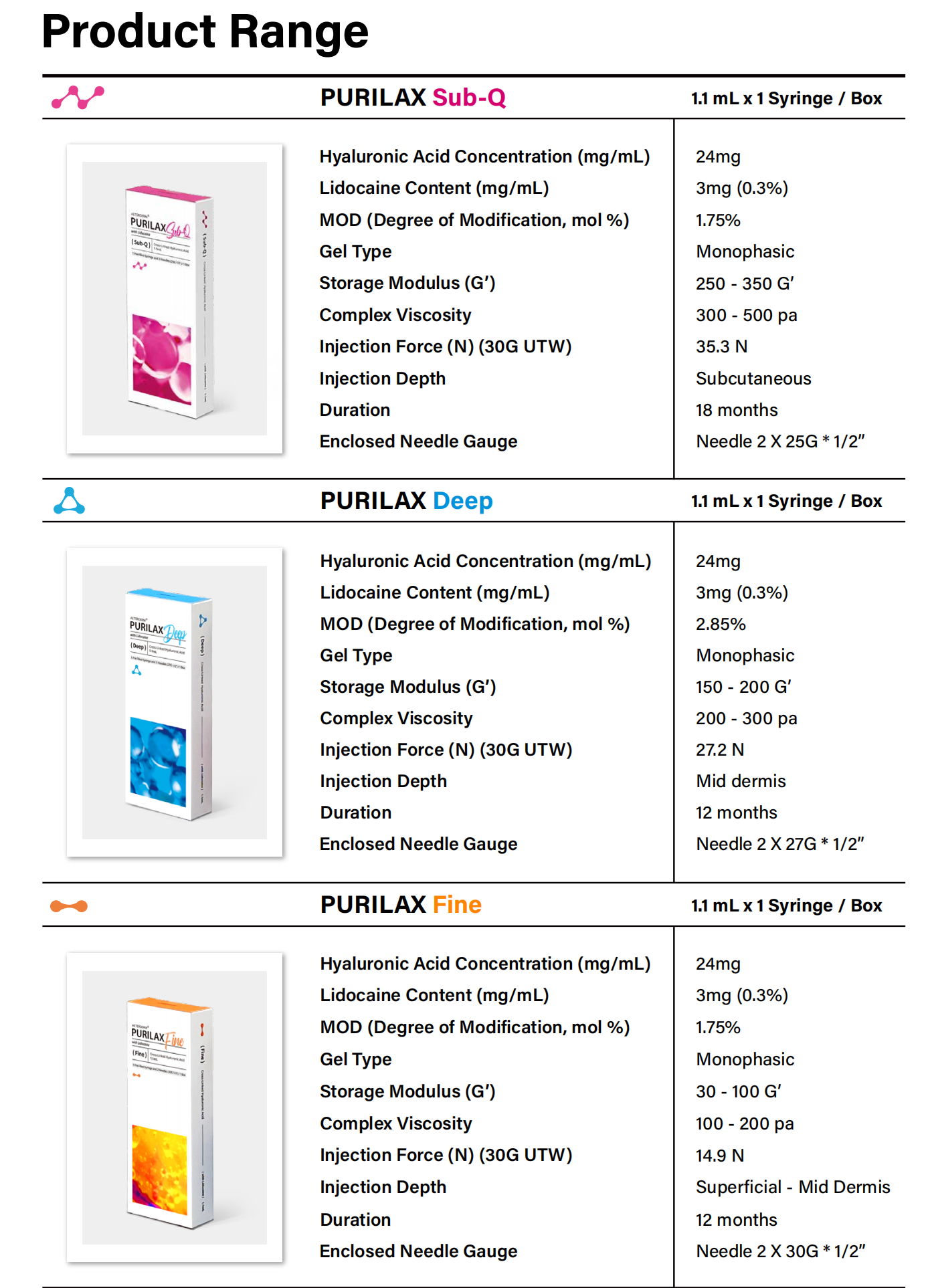
Product Specifications
- HA Concentration: 24mg/mL ± 10%
- Cross-linker: Zero BDDE technology
- Needle Options: 30G×½” / 27G×1¼”
- Volume: 1.0mL pre-filled syringe
- pH Balance: 7.2-7.6 physiological range
- Storage: 2-25°C in original packaging
Clinical Protocol
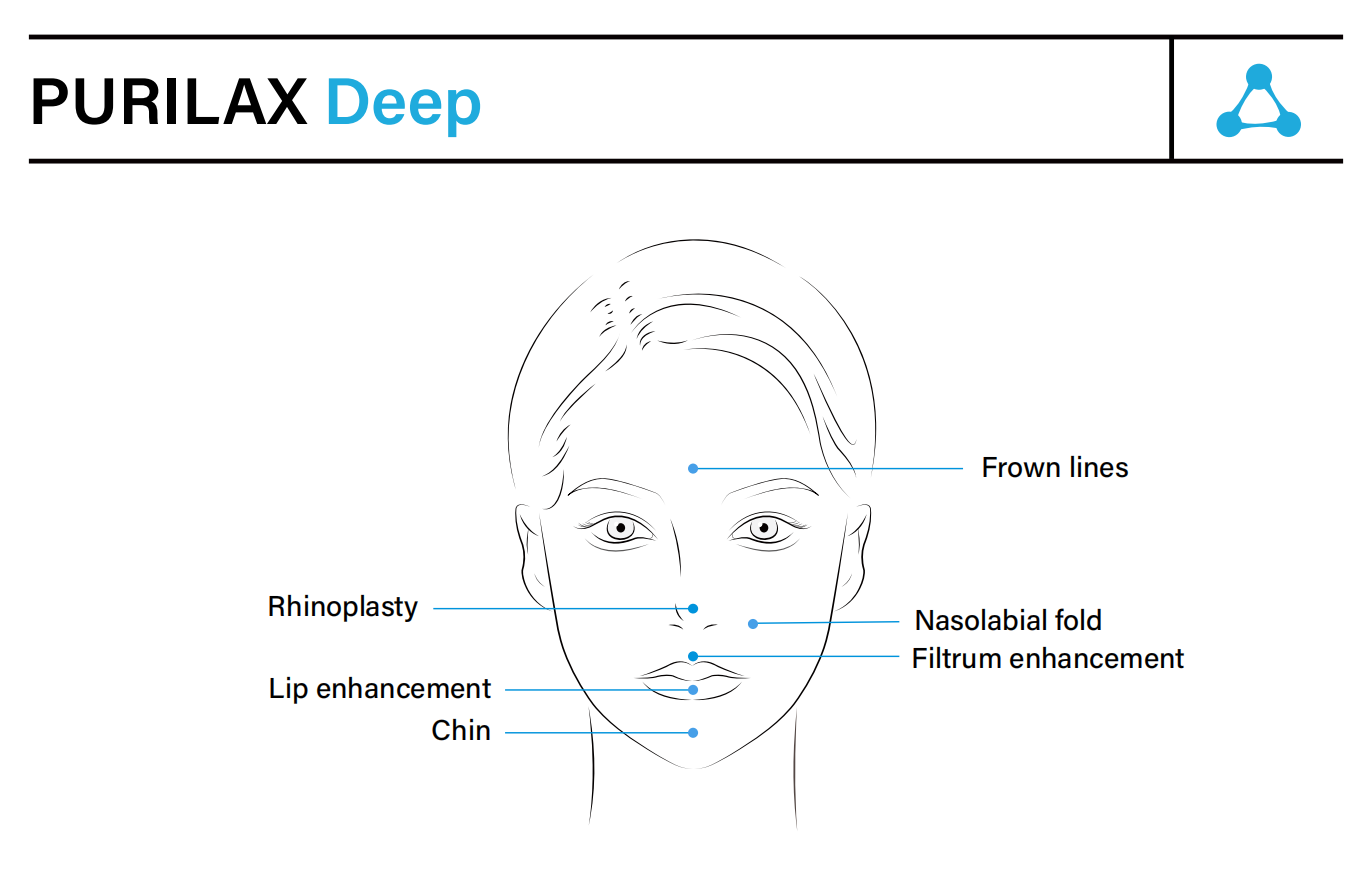
- Indications: NLF, marionettes, lip augmentation
- Injection Depth: Mid-to-deep dermis (Purilax Fine)
- Technique: Serial puncture or linear threading
- Max Dose: 2.0mL per treatment session
- Anesthesia: Integrated 0.3% lidocaine
Therapeutic Applications
Mid-Dermal Rejuvenation
For perioral rhytides: 27G needle at 30° angle with 0.02mL depot injections. Maintain 85% correction rate at 9-month follow-up.
Volumetric Restoration
Malar augmentation: Cannula delivery with 1.2-1.8mL per side. 3D imaging shows 12-month projection maintenance.
Lip Enhancement
Vermilion border definition: 30G needle with feathering technique. 92% patient satisfaction in 180-day multicenter study.
Medical Practitioner Advisory
Notice: Individual results may vary based on injection technique and tissue characteristics. Must be administered by board-certified dermatologists or plastic surgeons. Contraindicated in patients with hypersensitivity to lidocaine or active skin infections.
Post-treatment monitoring required for vascular complications. Not intended for glabellar or nasal dorsum applications. Always aspirate before injection. Report adverse events to local regulatory authority.












Reviews
There are no reviews yet.